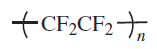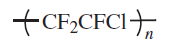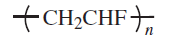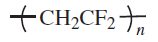Polyfluoroolefins (Fluoropolymers)
Properties
Fluoropolymers are produced from alkenes in which one or more hydrogen atoms have been replaced by fluorine. The most important members of this polymer class are polytetrafluoroethylene (PTFE), polychlorotrifluoroethylene (PCTFE), poly(vinyl fluoride) (PVF) and poly(vinylidene fluoride) (PVDF)
Polyfluoroolefins have a low coefficient of friction and low surface tension due to the weak van der Waals forces. They also have outstanding chemical, high temperature and weathering resistance due to the stability of the (multiple) carbon-fluorine bonds, which increases with the number of fluorine atoms in the repeat unit.
The largest-volume polyfluoroolefin is polytetrafluoroethylene (PTFE). This polymer has unique performance properties. It has outstanding thermal, electrical and chemical resistance, and can be used both at very high (up to 530 K) and extremely low temperatures. Its coefficient of friction is among the lowest of all polymers (self-lubricating and non-stick). PTFE cannot be dissolved in any common solvent below its melting point and is stable even in concentrated acids and bases. It is ideal for applications where broad chemical resistance, high durability, wide service temperature range, excellent dielectric properties, low friction, and non-stick are required. The properties of PTFE - high crystallinity, very high melting point (600 K), and very high melt viscosity - do not allow its processing by the usual melt-processing methods for plastics. Instead, similar to metal powder forming, the granular resins are processed by compression molding at ambient temperature followed by sintering above the crystalline melting point.
Various copolymers of tetrafluoroethylene (PFA, FEP, ETFE) and other fluoropolymers with lower melting point and crystallinity were developed to overcome the lack of melt processability of PTFE. Among these, poly(vinylidene fluoride) (PVDF) is noteworthy. This polymer is one of the easiest to process fluoropolymers. It has high tensile and impact strength, and excellent resistance to tensile creep and fatigue and, like PTFE, it exhibits high thermal stability. However, its chemical resistance is not as good as that of PTFE. For example, it is attacked by strong bases, amines, esters, and ketones. PVDF undergoes crosslinking when exposed to ionizing radiation which allows for the modification of its mechanical and thermophysical properties. Furthermore, it is (partially) compatible with a number of other resins including acrylics and methacrylics and acrylic rubbers (ACM).
Another important fuoropolymer is polyvinylfluoride (PVF). It is a semicrystalline, transparent to opaque thermoplastic polymer which has excellent weatherability, outstanding mechanical properties, and is inert towards a large number of common chemicals and solvents. The degree of crystallinity can vary considerably which greatly affects its mechanial properties. Films made from PVF are strong, flexible and have good fatigue-resistance and can be used for applications in the temperature range from approximately 200 K to 380 K.
COMMERCIAL FLUOROPOLYMERS
The commercial production of fluoropolymers is relativly small when compared to commodity polymers such as polyethylene, polypropylene or PVC, but still significant in comparison to many other specialty engineering polymers. The global demand on fluoropolymers was approximately 7 billion USD in 2011 (Source: Market Report - Global Fluoropolymer Market. Acmite Market).
Examples of Fluoropolymers
| Fluoropolymer | Repeat Unit | Manufacturer | Trade Name | ||||||||||||||||
| Polytetrafluoroethylene (PTFE) |
| DuPont, 3M, Solvay, AGC |
Teflon®,
Dyneon™ ,
Fluon®,
Hostaflon, Chemfluor,
Algoflon®
| Polychlorotrifluoroethylene (PCTFE) |
| Solvay, SABIC (3M), Daikin |
Halar® ECTFE,
Kel-F®1,
Neoflon™,
Halon®, Aclon®, Aclar® | Poly(vinyl fluoride) (PVF) |
| DuPont |
Tedlar® | Poly(vinylidene fluoride) (PVDF) |
|
Solvay, Arkema |
Hylar® PVDF,
Kynar®,
Tecaflon® | |
APPLICATIONS
Fluoropolymers find numerous applications in the electrical (coaxial cables for radio frequency, tapes, seat heating, appliance and aircraft wiring), chemical (lined pipe and fittings, gaskets, thread sealant tapes, filters, membranes), and mechanical industry (bearings, seals, piston rings, anti-stick coatings, self-lubricating parts).
PTFE is the most important but also most expensive fluoropolymer. It is used for applications where broad chemical resistance, high durability over a wide service temperature range, excellent dielectric properties and a low coefficient of friction are required or are advantageous. Noteworthy applications include low-friction bearings, gears and slide plates; chemical resistant valves, pump parts, filters and membranes; non-stick coatings for moulds, dies, and cookware; electrical insulators in connector assemblies, cables and in printed circuit boards.
PVDF is used in the aircraft, electronics, architectural coating and chemical industry. Important applications include chemical resistant valves, bearings, pump parts, and heat-shrinkable tubing. It is also used in the specialty packaging industry for agricultural and industrial films and for certain food packaging. Because of its higher cost, PVDC is often combined with other cheaper plastics or it is applied as a thin coating or laminate, for example to improve the chemcial resistance and weatherability of metals such as aluminum, and galvanized steel.
PCTFE is mainly used as moisture protective film or coating in pharmaceutical blister packaging due to its excellent water vapor barrier properties and good chemical stability. Other applications include chemical resistant tubes, valves, tank liners, O-rings, seals and gaskets.
PVF is mainly used as a surface protecting laminate in the aircraft and architectural industry to improve the chemical and UV resistance, and to provide an easy-to-clean surface. Important film applications include wall coverings, residential and commercial roofing, siding, air-inflated structures, canopies, awnings and stadium domes.